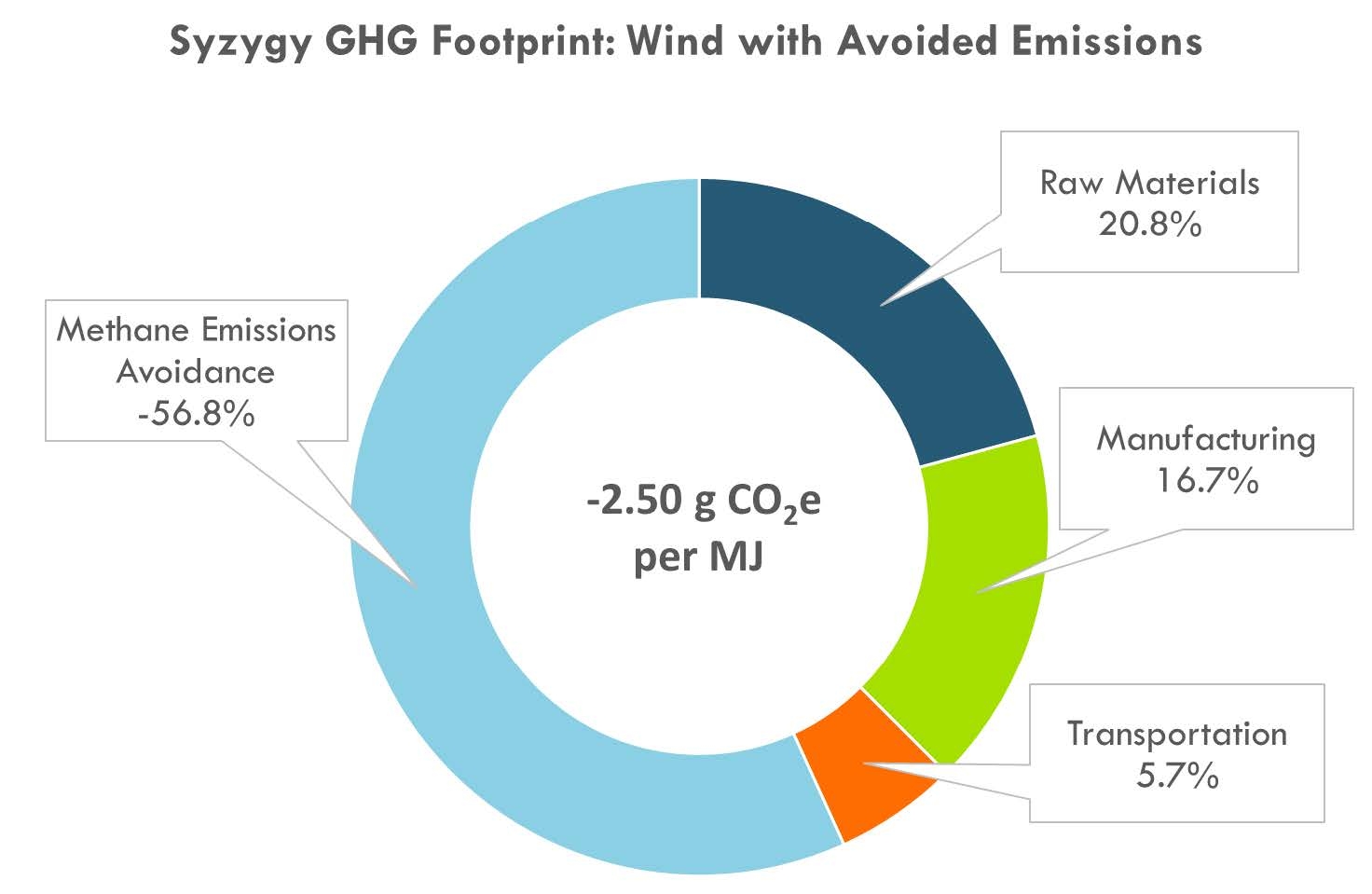
In a recent lifecycle analysis report, Boundless Impact Research & Analytics compares Syzygy's sustainable aviation fuel (SAF) technology to competing aviation fuels, including Jet A, Power-to-Liquid (PtL), and ethanol-based Alcohol-to-Jet (ATJ-e).
The study concludes that Syzygy SAF has a GHG footprint of -2.50 g CO2e/MJ when utilizing biogas and wind energy, giving it the lowest carbon footprint compared to other SAF pathways.
GHG Footprint analysis is based on a well-to-wake system boundary.
The environmental performance reflects all upstream and downstream impacts from raw material extraction and transportation to fuel production and combustion. This assessment does not account for environmental impacts from Syzygy's facility infrastructure or product packaging, following alignment with industry-standard Life Cycle Assessment (LCA) methodology.
Hydrogen has many uses across industries. It is an integral component in refining petroleum, producing fertilizer, producing things like steel and glass, and even in treating foods. And of course there is the push to use it as an alternative fuel source in heavy transport and maritime shipping where battery size and weight reduce the volume of cargo you can carry.
It might surprise some to learn that there is an entire rainbow used to describe how hydrogen is produced. And most people have no idea what any of those colors actually mean. So we’re going to break that down. To skip straight to the green vs. blue section, scroll to the bottom of the page.
Syzygy hydrogen is off the color wheel entirely — Focus on carbon intensity instead of color

It might surprise some to learn that there is an entire rainbow used to describe how hydrogen is produced.
With the ability of our Rigel photoreactors to use renewable electricity to power the lights needed to cause chemical reactions, Syzygy has a way to convert green ammonia into hydrogen and nitrogen while achieving a lower carbon intensity score. While using the photocatalytic method described here results in zero emissions, the produced hydrogen does not technically qualify as “green hydrogen” according to current conventions because it does not use electrolysis.
Just like white was recently added to the ever-changing hydrogen color wheel, perhaps it is time for a tenth color. Or perhaps carbon intensity scores will take over entirely for the ever-shifting hydrogen color wheel.
The nine colors of hydrogen
Until recently there were eight identified colors of hydrogen. But the addition of white brings the current count to nine.
There are strong arguments being made by government agencies and industry representatives to step back from the colors of hydrogen to realign the classification system based on carbon intensity, or CI. Carbon intensity refers to the weight of carbon equivalent emissions produced per unit of energy consumed or substance produced. For our purposes, CI includes everything from the carbon dioxide released while producing hydrogen (net of carbon capture) to upstream feedstock and electricity emission to what it takes to transport it and store it—everything from cradle to grave. Syzygy has even built a
carbon model calculator to help our customers better understand their full CI picture. But until we have widespread agreement on how to factor CI, the hydrogen world is stuck with the color wheel.
White hydrogen —The newest addition to the color wheel, white hydrogen is geological and naturally occurring. While found underground in natural deposits, we have limited ways to harvest and use this potentially CI-friendly form of hydrogen.
Black hydrogen — This is one of the worst offenders on the carbon intensity scale. It’s the bad guy. Black hydrogen is produced by heating coal, which we’ve recently heard referred to as carbon ore (again, no kidding), to temperatures as high as 1,292°F (700°C) to break down the organic carbonic materials. Steam is used to keep the coal from combusting and the coal breaks down into hydrogen, carbon dioxide, and carbon monoxide. The hydrogen is collected while the CO2 and CO get released into the atmosphere, giving black hydrogen an extremely high CI score.
Brown hydrogen — Another of the worst offenders on the CI scale, brown hydrogen is called that only because it comes from lignite, which is coal that happens to be brown. So its carbon intensity score is just as high as black hydrogen.
Grey hydrogen — Accounting for over 90 percent of hydrogen production around the world, grey hydrogen is produced using a process called steam methane reforming (SMR). In this process, methane from natural gas reacts with high-temperature steam to produce hydrogen, carbon monoxide, and carbon dioxide. This represents the carbon intensity status quo we are trying to improve, where carbon dioxide and carbon monoxide are both freely released into the atmosphere. The main reason for the high CI score for grey hydrogen is the process energy for SMR comes from fossil fuel combustion. All that combustion results in a lot of emissions. This is why Syzygy is so excited about the possibility of
using light instead of heat to produce hydrogen.
Turquoise hydrogen — Using a process termed pyrolysis, methane decomposes at very high temperatures to generate hydrogen and solid carbon. While pyrolysis produces no carbon dioxide, it again requires incredibly high temperatures. New technologies have plans to replace the heat from combustion with electrical heating which helps the CI score. And While the carbon intensity score of turquoise hydrogen is lower than some other colors on the wheel, the production process is new and has yet to scale.
Yellow hydrogen —This is hydrogen produced using electrolysis, which uses electricity to separate water molecules into hydrogen and oxygen. It is called yellow because producers use any available electricity, as opposed to using wind or solar renewable electricity exclusively. The carbon intensity score for yellow hydrogen could potentially be higher than that for black, brown, and grey hydrogen, and is definitely not not as good as blue or green.
Pink hydrogen | purple hydrogen | red hydrogen — Depending on where you look, you will sometimes see references to pink, purple, or red hydrogen. They are all talking about the same thing. This is hydrogen produced from electrolysis where electricity in the electrolyzer is produced from nuclear power. With zero carbon emissions associated with this process, the carbon intensity score for pink, purple, and red hydrogen is excellent. However, current social, political, and safety concerns about nuclear power plants make these colors on the wheel less than attractive.
Blue hydrogen — While blue hydrogen is produced with steam methane reforming from natural gas or coal, it incorporates the capture and storage of carbon dioxide to improve its CI score. Carbon capture and sequestration (CSS) and carbon capture, utilization, and sequestration (CCUS) both call for collecting the carbon dioxide produced during the SMR process and storing it long-term in underground storage facilities. Blue hydrogen is promising in that it offers a low-carbon way to produce hydrogen under existing definitions. It can even be carbon negative if it uses renewable natural gas (waste gas which can be captured from landfills, agriculture operations, and wastewater treatment plants that would otherwise be emitted into the atmosphere). The biggest problem with blue hydrogen is finding proper storage near the hydrogen production plant. Without the right geological formations, storing carbon gases long term underground is a non starter.
Green hydrogen — Using electrolyzers and electricity exclusively from renewable sources (wind, solar, hydropower, etc.), green hydrogen has the best carbon intensity score of all the hydrogens on the color wheel. The problem is that green hydrogen production costs are at least 30 percent higher than blue hydrogen. Until the cost of green hydrogen comes down, it will struggle to gain adoption.
Olefins are widely used as raw materials in the manufacture of chemical and polymer products like plastic, detergent, adhesive, rubber, and food packaging. They consist of a group of chemicals: ethylene, propylene, and butadiene.
How they are made
There are multiple methods for producing olefins from crude oil, natural gas, coal, and methanol. These petrochemical processes break down saturated hydrocarbons into unsaturated hydrocarbons. The unsaturated hydrocarbons (ethylene, propylene, and butadiene) can then be used to manufacture a wide range of essential products.
The takeaway is that all olefin production currently uses hydrocarbons as the base starting substance and requires heat from burning fossil fuels throughout the process. The processes needed to manufacture are considered hard to abate because the limiting factors are defined by cost and the laws of chemistry.
Olefins and the products they give us
Olefins are widely used to manufacture food packaging and essential products.

Olefins are widely used to manufacture food packaging and essential products.
Ethylene
Ethylene is the most widely produced of the olefins and is used across many industries. It is a basic ingredient in things like fabricated plastics and antifreeze, and is used to make fibers. It is the foundation for ethylene oxide, most widely used as a sterilizing agent for medical equipment and in manufacturing ethylene glycol. And it is the primary building block for producing polyethylene, used in food packaging, grocery bags, wire insulation, toys, mulch, and multiple kitchen and household products.
Propylene
Propylene is also called propene and methyl ethylene. Its primary use is in making polypropylene, a material that accounts for 25% of all plastic products. Polypropylene is present in things like food packaging films, prescription bottles, plastic parts, carpet fibers, and some textiles.
Other applications include:
- propylene oxide (used in fumigants, detergents, and lubricants)
- acrylic acid (found in coatings, adhesives, elastomers, paints, and polishes)
- acrylonitrile (rubber and some plastics)
- cumene (paint thinner)
Butadiene
The main applications for butadiene involve synthetic rubbers. It is in things like tires, carpet backing, gloves, shoe soles, wetsuits, and asphalt. Butadiene is a primary component in neoprene fabric.
Reducing the negative impact of olefins on the environment
While olefins by definition are a derivative of hydrocarbons, a significant volume of fossil fuel must be burned throughout the chemical processing chain to supply heat to fire the conventional chemical reactors. Replacing dirty fossil-fuel-fired chemical reactors with photoreactors will dramatically reduce greenhouse gas emissions related to olefins.
While lower-emission olefin production will be difficult, it is on the Syzygy research and development roadmap.